TubeClear Receives FDA Clearance for New GJ-Specific Clearing Stem Model
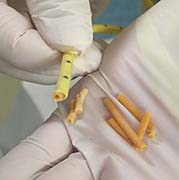
BELLEFONTE, PA – Actuated Medical, Inc. (AMI) recently received its sixth Food and Drug Administration (FDA) 510(k) clearance for the TubeClear® system. Clearance number K200646 introduces a Clearing Stem model that is compatible with select gastro-jejunostomy (GJ) feeding tubes.
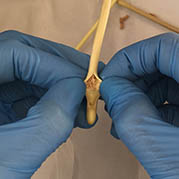
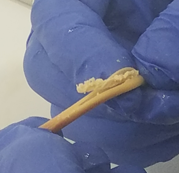
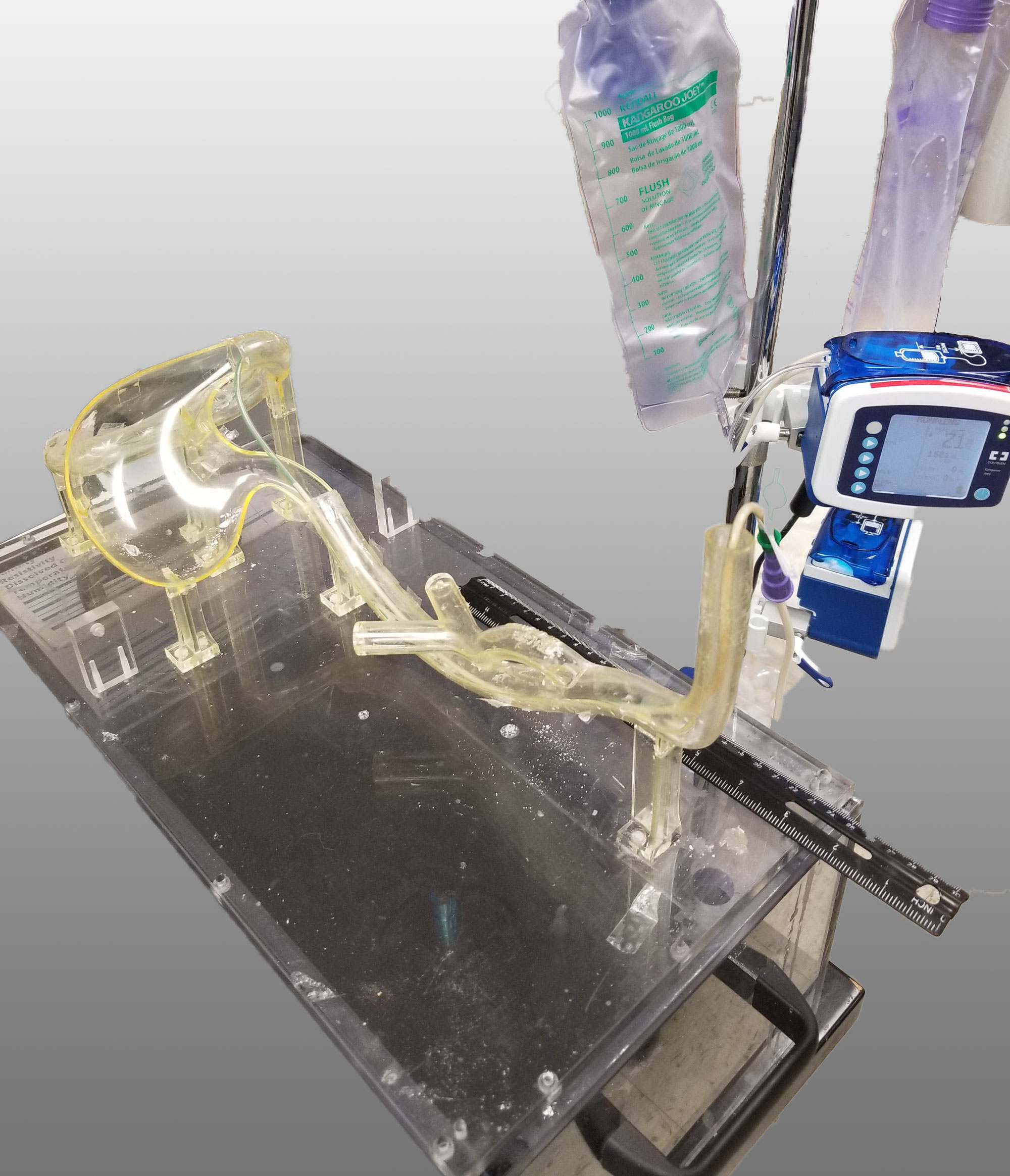
These complex dual lumen feeding tubes are often expensive and require surgery to place. When a clogged feeding tube of any type cannot be cleared, it requires replacement. The new TubeClear GJ-1422 Clearing Stem offers a mechanical option for clinicians to keep these expensive GJ tubes clear and avoid an unnecessary procedure to replace the tube. The GJ-1422 Clearing Stem is designed to work with MIC*, MIC-KEY* (Avanos Medical, Inc., Alpharetta, GA) and G-Jet® (Applied Medical Technology (AMT), Brecksville, OH) GJ feeding tubes that are 14-22 French diameter and have a jejunal length of 15-45 centimeters.
“Clogging in GJ tubes is one of the most common complaints we hear from clinicians and patients alike,” said AMI president and chief executive officer Maureen L. Mulvihill, PhD. “Being able to deliver a Clearing Stem that is compatible with our existing TubeClear system as well as GJ feeding tubes is an exciting opportunity for AMI to support vulnerable patients and improve their outcomes.”
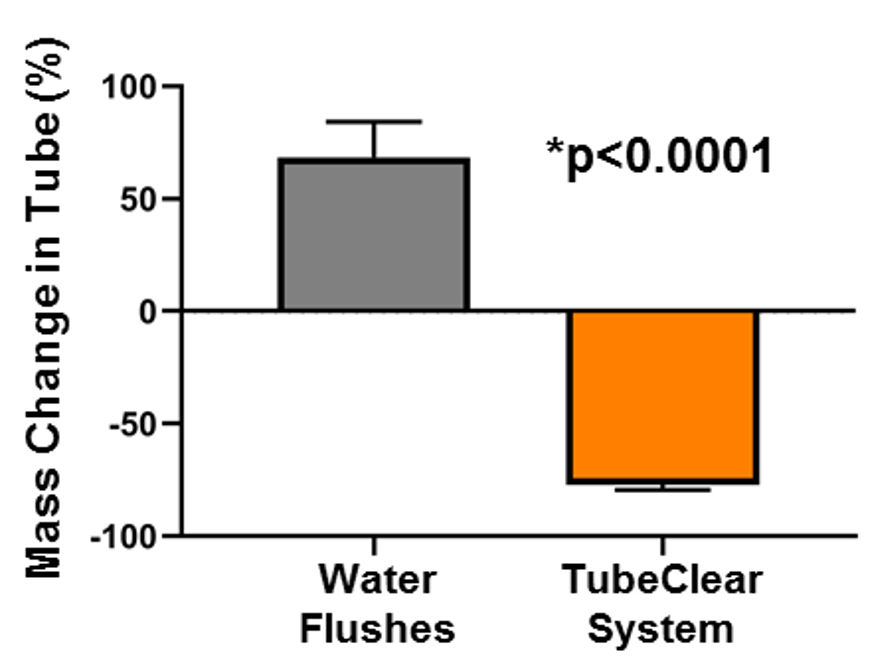
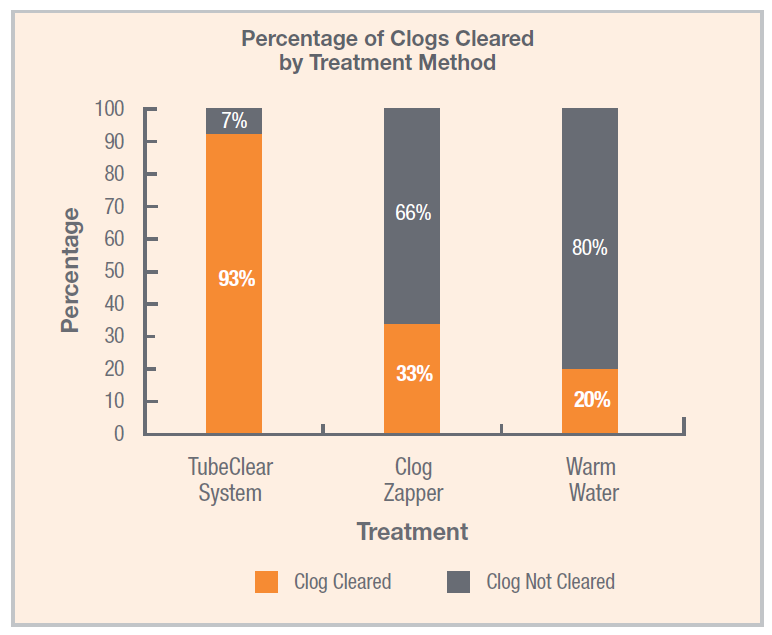
The TubeClear system is an FDA cleared medical device that uses patented mechanical motion technology to maintain flow and clear clogs in most feeding and decompression tubes while the tube remains in the patient and works at bedside. Keeping these tubes clear prevents interruptions to feeding and medication delivery, allowing clinicians to maintain their focus on improving patient care and reducing healthcare cost associated with tube replacement. The system, which is comprised of reusable control boxes and single-use clearing stems, is designed, developed, and manufactured by AMI in Bellefonte, PA. The new GJ Clearing Stem is slated to be available for sale in clinical settings in early 2021.
***
About Actuated Medical, Inc.
Actuated Medical develops medical devices that integrate electronically controlled motion technologies that improve patient outcomes and reduce healthcare costs. Their development process focuses on intellectual property, regulatory, and reimbursement strategies to decrease commercialization risk and attract medical device technology licensing partnerships. Their devices solve unmet clinical needs in target markets (e.g., GI, oncology, critical care and pediatrics). Actuated Medical a certified women-owned business located in Bellefonte, PA and is ISO 13485:2016 certified.
For more information, please visit http://www.actuatedmedical.com