Dec 4, 2020 | News, Press Releases
BELLEFONTE, PA – Actuated Medical, Inc. (AMI) recently received its sixth Food and Drug Administration (FDA) 510(k) clearance for the TubeClear® system. Clearance number K200646 introduces a Clearing Stem model that is compatible with select gastro-jejunostomy (GJ)...
Mar 2, 2020 | Information
No one wants a clogged feeding tube. They impact the patient’s ability to receive adequate nutrition and keep medication on schedule.[1] If the clog cannot be cleared, the tube is replaced. Tube replacement can be a painful, risky, and costly procedure that can...

Jan 9, 2020 | Bench Study
Enteral nutrition (EN) is provided for patients that have a functioning lower gastrointestinal (GI) tract but are unable to orally ingest nutrients and medication and are at risk for malnutrition.[1] Feeding tubes (Tubes) provide these patients essential nutrition,...
Dec 4, 2019 | Abstracts & Posters
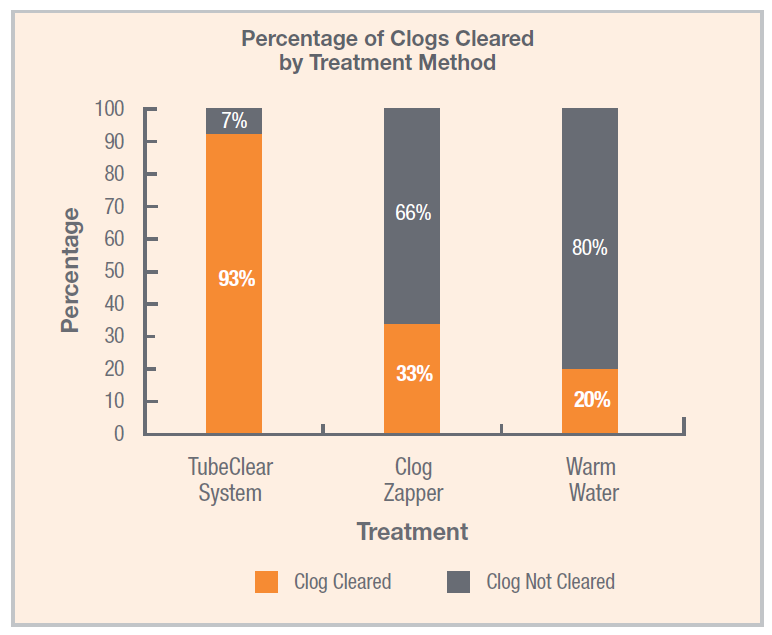
In a recent study published in Nutrition in Clinical Practice by Christopher M. Garrison, Ph.D., RN, CNE, the TubeClear system was found to be significantly more effective at removing clogs from feeding tubes than water or enzyme-based clog-clearing treatments.[1]...
Sep 30, 2019 | Bench Study
Many people think feeding tubes are merely used to deliver nutrition (i.e., feeding formula), however, they deliver life-sustaining medications too – it’s no wonder they tend to clog. At Actuated Medical, Inc., we developed the TubeClear System to keep enteral...


