Dec 4, 2020 | News, Press Releases
BELLEFONTE, PA – Actuated Medical, Inc. (AMI) recently received its sixth Food and Drug Administration (FDA) 510(k) clearance for the TubeClear® system. Clearance number K200646 introduces a Clearing Stem model that is compatible with select gastro-jejunostomy (GJ)...
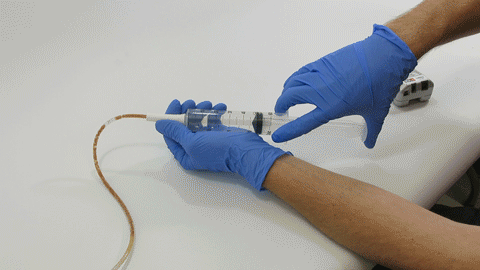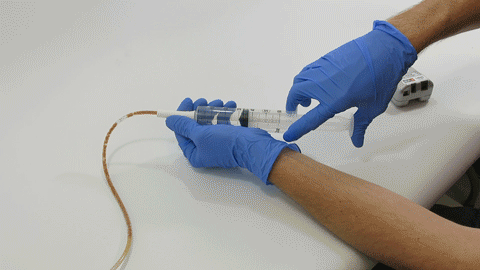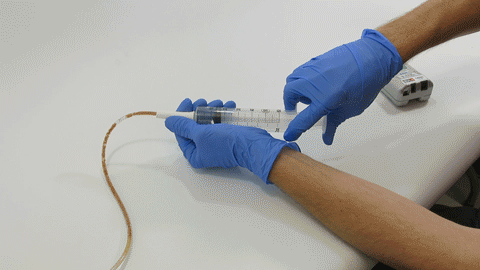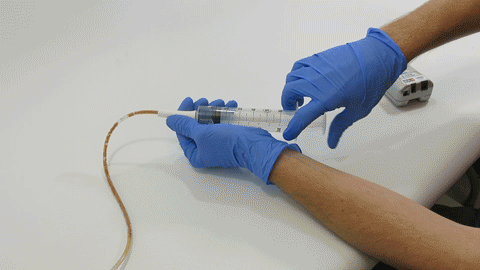
Sep 30, 2019 | Bench Study
Many people think feeding tubes are merely used to deliver nutrition (i.e., feeding formula), however, they deliver life-sustaining medications too – it’s no wonder they tend to clog. At Actuated Medical, Inc., we developed the TubeClear System to keep enteral...


