Dec 4, 2020 | News, Press Releases
BELLEFONTE, PA – Actuated Medical, Inc. (AMI) recently received its sixth Food and Drug Administration (FDA) 510(k) clearance for the TubeClear® system. Clearance number K200646 introduces a Clearing Stem model that is compatible with select gastro-jejunostomy (GJ)...

Jan 9, 2020 | Bench Study
Enteral nutrition (EN) is provided for patients that have a functioning lower gastrointestinal (GI) tract but are unable to orally ingest nutrients and medication and are at risk for malnutrition.[1] Feeding tubes (Tubes) provide these patients essential nutrition,...
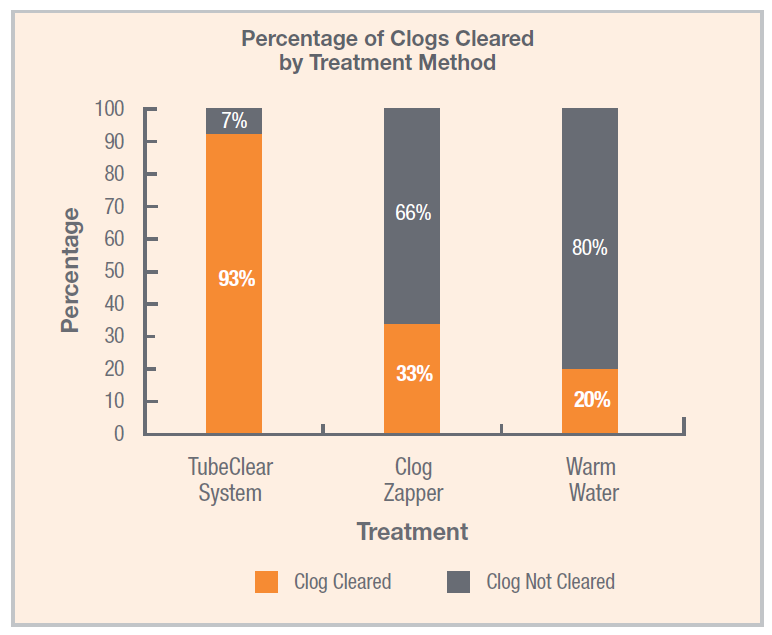
Dec 4, 2019 | Abstracts & Posters
In a recent study published in Nutrition in Clinical Practice by Christopher M. Garrison, Ph.D., RN, CNE, the TubeClear system was found to be significantly more effective at removing clogs from feeding tubes than water or enzyme-based clog-clearing treatments.[1]...

