Jan 17, 2017 | Press Releases
Building on its strong foundation, Actuated Medical hires Sales Representatives for sales expansion of the TubeClear System
BELLEFONTE, PA – January 17, 2016 – Maureen L. Mulvihill, President and CEO of Actuated Medical, Inc.(AMI) announces the addition of 6 senior sales leaders to support the nationwide sales launch of the TubeClear® System. These sales leaders were formerly part of the top producers for a recently acquired medical device company. Repeatedly recognized for their performance, they have a proven track record of exceeding customer expectations and establishing relationships with key customer constituencies.
“I have the great confidence that these Sales Leaders. Not only will they assist healthcare organizations in more effectively and efficiently clearing clogs in feeding and decompression tubes, but they will also be able connect with clinicians to identify new clinical needs. We are always looking for the next device to develop that improves patient outcomes and these leaders will be our eyes and ears with our customers,” says Mulvihill.
Using patented technology platforms, AMI develops medical devices that have unmet clinical needs in targeted markets. AMI has multiple devices in development that have been funded using non-equity capital (e.g., Small Business Innovation Research (SBIR) funds). For each technology, the goal is to develop devices for acquisition by a strategic buyer or private equity group. AMI’s first product, the TubeClear System is already FDA cleared and is building sales revenue to attract a technology acquisition.
The sales representatives along with Joseph Kroll, Director Clinical Education, will support TubeClear System customers nationwide. “These sales leaders understand the challenges faced by the nursing staff and nutritional support specialists. They are passionate about not only expanding the TubeClear System’s usage, but also about improving patient outcomes,” says Kroll.
About Actuated Medical, Inc.
Actuated Medical Inc. is committed to improving patient outcomes by designing the next generation of innovative and advanced medical devices through continuous improvement and innovative solutions.
US-based Actuated Medical develops, and manufactures minimally invasive medical devices to enhance clinical care and deliver positive patient outcomes. Actuated Medical a certified women-owned business located in Bellefonte, PA and is ISO 13485:2003 certified.
Dec 7, 2016 | Press Releases
TubeClear System cleared for Smaller Feeding and Decompression Tubes
BELLEFONTE, PA – December 7, 2016 – Actuated Medical Inc. (AMI) received U.S. Food and Drug Administration (FDA) Clearance on 3 additional TubeClear System Clearing Stem Models. This FDA clearance enables the TubeClear System to operate in feeding tubes as narrow as 6 French (2mm), which expands the capability of the TubeClear System to gastrostomy (G), jejunostromy (J) and nasoenteral (NE) feeding and decompression tubes between 6 and 18 French in adult patients. The FDA clearance also enables licensed or certified healthcare practitioners to use the TubeClear System to clear the clogged feeding tubes at a patient’s bedside.
Maureen L. Mulvihill, AMI’s President and CEO stated that, “We are excited by the additional FDA clearance. The ability to clear clogs in smaller diameter feeding tubes opens new US and international markets. It also enables paramedics to potentially clear clogged tubes in the patient’s home avoiding transportation and hospital readmission costs.”
Feeding and decompression tubes are used to supply nutrition and medication to patients that cannot self-feed. These tubes are narrow in diameter and clog at about a 25% rate (~1.7million clogs annually). Caregivers spend significant time trying to clear the clogged tubes. When a tube cannot be cleared, the tube must be replaced which means another costly, invasive procedure for the patient. (The costs of feeding tube replacement can be greater than $1,200 per instance when accounting for clinician time, effort and associated supplies.) The TubeClear System clears clogged feeding tubes at the patient’s bedside, while the tube remains in the patient. Comparison testing has shown that the TubeClear System works in minutes and is more effective than other methods used in the industry. It is expected that the replacement rate in environments where the TubeClear System is utilized will be significantly reduced.
“Nasogastric feeding tube insertion is a painful experience. Therefore clinicians often use smaller diameter feeding tubes for comfort and/or to accommodate a patient’s size. With this FDA clearance, AMI offers a solution to clear smaller diameter tubes at the patient’s bedside. The idea of reducing patient pain and unnecessary invasive procedures is what drives us, at AMI, to continue to innovate.” said Joe Kroll, AMI’s Director Clinical Education.
The TubeClear system, which is comprised of reusable Control Boxes and single-use Clearing Stems, is designed, developed and manufactured by Actuated Medical, Inc. in the United States. To learn more about the TubeClear System, additional information can be found at tubeclear.com.
About Actuated Medical, Inc.
Actuated Medical Inc. is committed to improving patient outcomes by designing the next generation of innovative and advanced medical devices through continuous improvement and innovative solutions.
US-based Actuated Medical develops, and manufactures minimally invasive medical devices to enhance clinical care and deliver positive patient outcomes. Actuated Medical a certified women-owned business located in Bellefonte, PA and is ISO 13485:2003 certified. For additional information, please visit www.actuatedmedical.com.
Dec 1, 2015 | Bench Study
Small bore feeding tubes are used to provide essential nutrition and medication to patients at risk of malnutrition and dehydration due to an inability to ingest orally (1).
Clogging is one of the most frequent mechanical complications of feeding tubes (2,3). Tubes are more likely to become clogged when powdered, crushed, acidic, or alkaline medications or ground feeding formulas containing particulates are delivered through the small inner lumen, or when tubes are not routinely flushed following feedings (2). Reported clogging rates vary, ranging from 9-35% (1, 2).
 Attempts to clear clogged feeding tubes using various techniques are time-consuming and often result with tube replacement still being required. Also, many of the current methods only work on certain types of clogs. For example, most enzyme treatments are only applicable to protein (feeding formula) based clogs. Among common emergency room medical procedures, patients rank nasoenteral (NE), (nasogastric [NG]) tube insertion to be one of the most painful (4, 5, 6). Replacing a feeding tube may cause additional patient safety risks, associated medical costs, and additional pain and discomfort to the patient. The most common way to place NE (NG) feeding tubes, is blind insertion at patient bedside, and has a reported 0.5-15% malposition rate (3). Malposition into the trachea may cause pneumothoraxes and possibly death. Several methods exist for verifying accurate tube placement, the most reliable being radiography (3). However, this exposes patients to additional radiation and medical costs.
Attempts to clear clogged feeding tubes using various techniques are time-consuming and often result with tube replacement still being required. Also, many of the current methods only work on certain types of clogs. For example, most enzyme treatments are only applicable to protein (feeding formula) based clogs. Among common emergency room medical procedures, patients rank nasoenteral (NE), (nasogastric [NG]) tube insertion to be one of the most painful (4, 5, 6). Replacing a feeding tube may cause additional patient safety risks, associated medical costs, and additional pain and discomfort to the patient. The most common way to place NE (NG) feeding tubes, is blind insertion at patient bedside, and has a reported 0.5-15% malposition rate (3). Malposition into the trachea may cause pneumothoraxes and possibly death. Several methods exist for verifying accurate tube placement, the most reliable being radiography (3). However, this exposes patients to additional radiation and medical costs.
Taking into account nursing time, tube replacement, radiographs and other miscellaneous costs, the capability to clear a clogged feeding tube while it remains in the patient, could represent substantial savings to a medical facility not to mention reduced pain and discomfort to the patient. For patients outside the hospital, a clogged feeding tube often results in transportation and admission costs to the payer and anxiety to the patient.
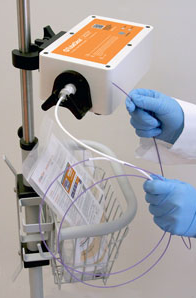
The TubeClear System was developed to efficiently and effectively clear clogged feeding tubes while the tube remains in the patient (7). It is currently FDA cleared to unclog 10-18 Fr NE, NG, gastrostomy (G [including percutaneous endoscopic gastrostomy, PEG tubes]) and jejunostomy (J) feeding tubes in adults at bedside while the tube remains in the patient. The TubeClear System is also CE marked. The TubeClear System is comprised of a reusable Control Box to which a single-use Clearing Stem is attached. The Clearing Stem is inserted into the patient’s feeding tube by the licensed healthcare practitioner. The Clearing Stem moves backward and forward, and the tip works against the clog to mechanically disrupt and clear the feeding tube.
+ FDA Cleared for clearing occlusions / clogs in Feeding and Decompression Tubes in adult patients that have a Tube size of 6 to 18 Fr.
References:
1. Ireton-Jones C, DeLegge M. Handbook of Home Nutrition Support. Sudburry, MA: Jones and Bartlett; 2007
2. Beyer PL, Matarese LE, Gottschlich MM. Complications of enteral nutrition. 1998.
3. Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20(26):8505-8524.
4. Singer AJ, Richman PB, Kowalska A, Thode HC, Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
5. Bae KH, Jeong IS. [Pain perception of nurses and pain expression of patients in critical care units]. J Korean Acad Nurs. 2014;44(4):437-445.
6. Penrod J, Morse JM, Wilson S. Comforting strategies used during nasogastric tube insertion. J Clin Nurs. 1999;8(1):31-38.
7. TubeClear System- Clears Clogged Feeding Tubes. 2015;http://www.tubeclear.com/
Dec 1, 2015 | Bench Study
Individuals unable to ingest nutrition and/or medication orally for various causes, often become dependent on enteral nutrition as a life-saving mechanism (1). However, due to daily administration of feeding formula and/or medication, as well as other unknown causes, these medically needed feeding tubes may become clogged, up to 35% of the time (1, 2).
Clogged feeding tubes often result in the interruption of a patient’s nutrition and medication regimens, representing a burden to both caregivers and patients. Attempts to clear clogged feeding tubes using various techniques are time-consuming and as a result cause delays in feeding, hydration, and medications which can have negative effects on overall patient health and healing (3). Often, these tube clearing methods fail and the tube requires replacement, exposing patients to both additional radiation from placement confirmation (x-rays) and increased medical costs. Notably, among common medical procedures, patients rank nasoenteral (NE), (nasogastric [NG]) tube insertion to be one of the most painful (4, 5, 6). The most common way to place NE (NG) feeding tubes, is blind insertion, and has a reported 0.5-16% malposition rate (7). Malposition into the trachea may cause pneumothoraxes and possibly death, though several methods exist for verifying accurate placement, the most reliable is radiography (7). Replacing clogged gastrostomy (G) and jejunostomy (J) feeding tubes may require additional endoscopic guidance or surgical procedures depending on tube type.
The TubeClear System, an FDA-cleared and CE marked medical device, was developed to clear clogged feeding tubes at bedside while the tube remains in the patient. The system comprises of a reusable Control Box that actuates a single-use Clearing Stem. The Clearing Stem inserts into the feeding tube and the backward and forward movement of the Clearing Stem acts to mechanically disrupt and clear the clogged feeding tube (8). It is currently FDA cleared to unclog 6-18 Fr NE, NG, G (including percutaneous endoscopic gastrostomy, [PEG tubes]) and J tubes while the tube remains in the patient.
+ FDA Cleared for clearing occlusions / clogs in Feeding and Decompression Tubes in adult patients that have a Tube size of 6 to 18 Fr.
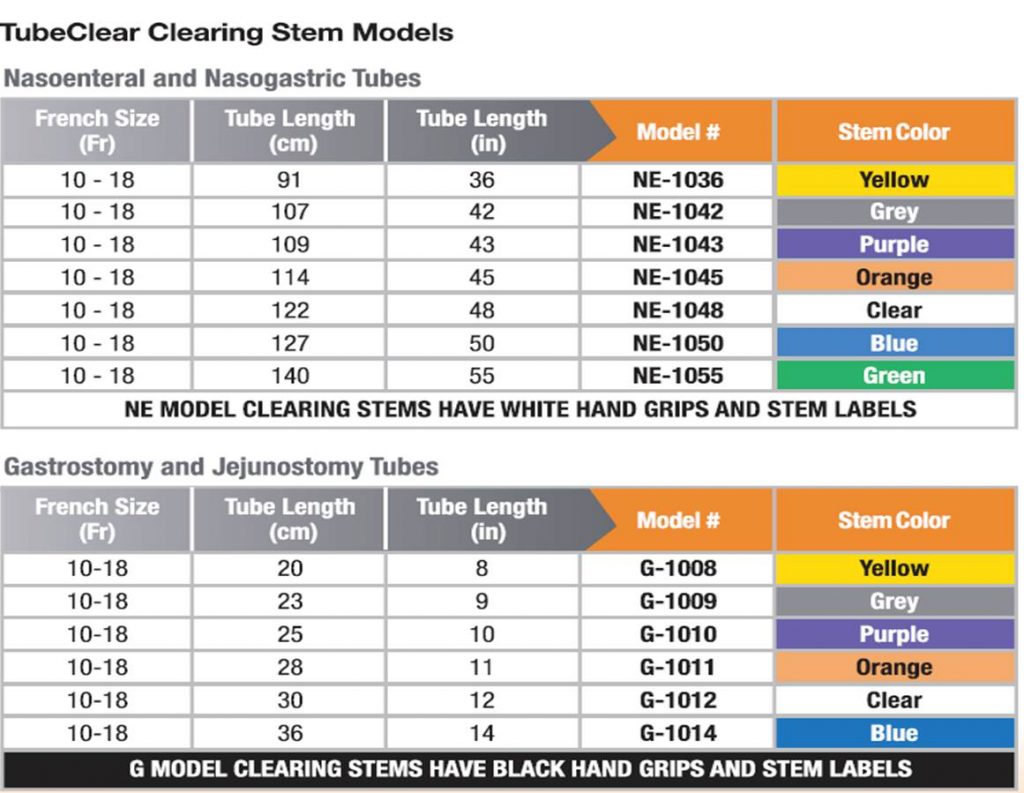
Using the TubeClear System to Clear Clogged G-tubes.
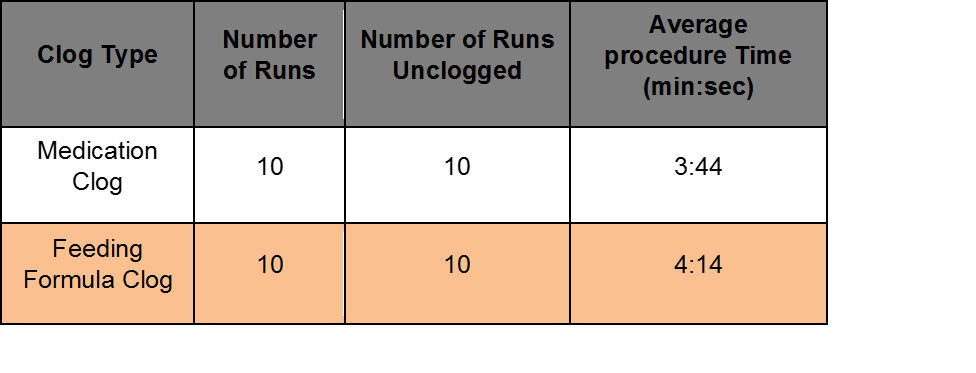
Benchtop Study evaluated at AMI with Levin 18 Fr, 14 inch long gastrostomy tubes. Half of the occlusions were made from 2:1 ratio of coagulated protein and ground medication (Medication Clog). Half of the occlusions were made from 1:1 ratio of feeding formula and fiber (Feeding Formula Clog).
Using the TubeClear System to Clear Clogged NE tubes.
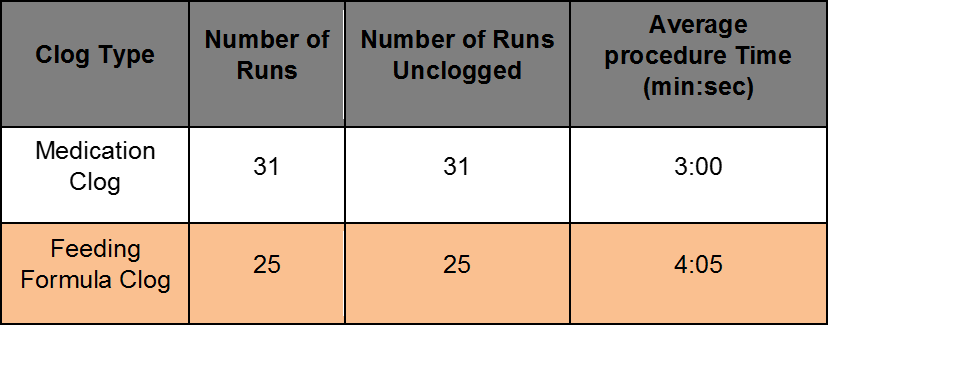
Benchtop Study evaluated at AMI with Levin 10F Fr, 55 inch long nasoenteral (Entriflex) tubes. Half of the occlusions were made from 2:1 ratio of coagulated protein and ground medication (Medication Clog). Half of the occlusions were made from 1:1 ratio of feeding formula and fiber (Feeding Formula Clog).
References:
1. Ireton-Jones C, DeLegge M. Handbook of Home Nutrition Support. Sudburry, MA: Jones and Bartlett; 2007
2. Beyer PL, Matarese LE, Gottschlich MM. Complications of enteral nutrition. 1998.
3. Pearce C, Duncan H. Enteral feeding. Nasogastric, nasojejunal, percutaneous endoscopic gastrostomy, or jejunostomy: its indications and limitations. Postgrad Med J. 2002;78(918):198-204.
4. Singer AJ, Richman PB, Kowalska A, Thode HC, Jr. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med. 1999;33(6):652-658.
5. Bae KH, Jeong IS. [Pain perception of nurses and pain expression of patients in critical care units]. J Korean Acad Nurs. 2014;44(4):437-445.
6. Penrod J, Morse JM, Wilson S. Comforting strategies used during nasogastric tube insertion. J Clin Nurs. 1999;8(1):31-38.
7. Blumenstein I, Shastri YM, Stein J. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20(26):8505-8524.
8. TubeClear System- Clears Clogged Feeding Tubes. 2015;http://www.tubeclear.com/
Dec 1, 2015 | Studies & Evaluations
Feeding tubes are used to deliver enteral nutrition to patients that are unable to ingest nutrients and medications orally and therefore are at risk of malnutrition and dehydration (1).
Unfortunately, the long and narrow inner lumens of small bore feeding tubes used to deliver enteral nutrition and medication to patients often clog for various reasons including, delivery of high viscosity medications, by chemical reactions between feeding formula and medication, or by inadequate tube maintenance (2,3). Clogged feeding tubes can cause patients to go extended periods without nutrition and medication, and attempts to unclog tubes strain
 valuable nursing resources. Traditional methods to clear clogged feeding tubes are often unsuccessful, potentially leading to tube replacement, and patient discomfort (2,3,4).
valuable nursing resources. Traditional methods to clear clogged feeding tubes are often unsuccessful, potentially leading to tube replacement, and patient discomfort (2,3,4).
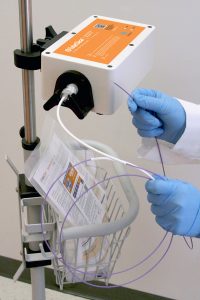
The TubeClear System, an FDA-cleared and CE marked medical device, was developed to clear clogged feeding tubes at bedside while the tube remains in the patient. The system comprises of a reusable Control Box that actuates a single-use Clearing Stem. The Clearing Stem inserts into the feeding tube and the backward and forward motion of the Clearing Stem Tip acts to mechanically disrupt and clear the clogged materials from the feeding tube (5).
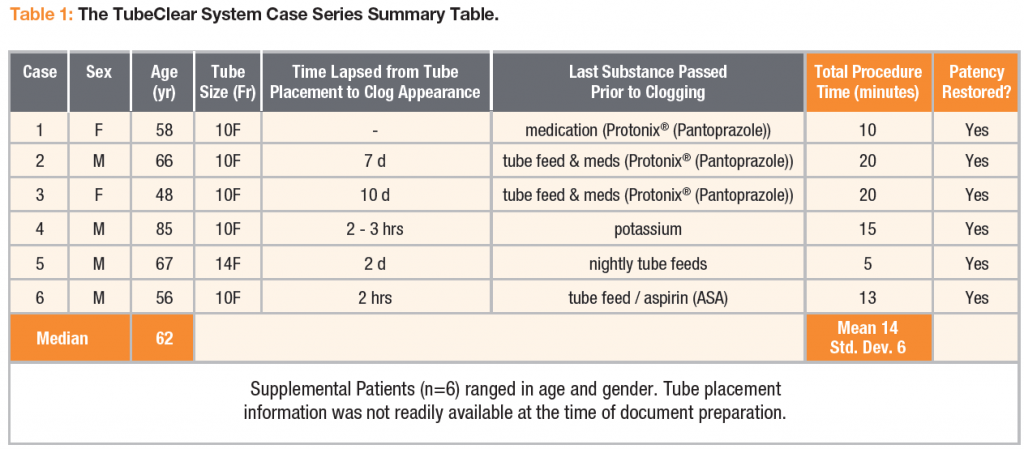
An in human case series was completed, documenting twelve cases in which TubeClear was used to clear clogged nasoenteral (NE) feeding tubes. All documented cases were completed by a single licensed healthcare practitioner and were 100% successfulwithout any issues reported by the practitioner and/or patients. The ability to quickly clear clogged feeding tubes while the tube remains in the patient will save healthcare resources, and ease the burden on patients and caregivers.
+ FDA Cleared for clearing occlusions / clogs in Feeding and Decompression Tubes in adult patients that have a Tube size of 6 to 18 Fr.
References:
1. Ireton-Jones C, DeLegge M. Handbook of Home Nutrition Support. Sudburry, MA: Jones and Bartlett; 2007
2. Beyer PL, Matarese LE, Gottschlich MM. Complications of enteral nutrition. 1998.
3. Metheny N, Eisenberg P, McSweeney M. Effect of feeding tube properties and three irrigants on clogging rates. Nurs Res. 1988;37(3):165-169.
4. Smith R, Myers S. 2 devices that unclog feeding tubes. RN. 2005;68(1):36-41; quiz 42.
5. TubeClear System- Clears Clogged Feeding Tubes. 2015;http://www.tubeclear.com/
 Attempts to clear clogged feeding tubes using various techniques are time-consuming and often result with tube replacement still being required. Also, many of the current methods only work on certain types of clogs. For example, most enzyme treatments are only applicable to protein (feeding formula) based clogs. Among common emergency room medical procedures, patients rank nasoenteral (NE), (nasogastric [NG]) tube insertion to be one of the most painful (4, 5, 6). Replacing a feeding tube may cause additional patient safety risks, associated medical costs, and additional pain and discomfort to the patient. The most common way to place NE (NG) feeding tubes, is blind insertion at patient bedside, and has a reported 0.5-15% malposition rate (3). Malposition into the trachea may cause pneumothoraxes and possibly death. Several methods exist for verifying accurate tube placement, the most reliable being radiography (3). However, this exposes patients to additional radiation and medical costs.
Attempts to clear clogged feeding tubes using various techniques are time-consuming and often result with tube replacement still being required. Also, many of the current methods only work on certain types of clogs. For example, most enzyme treatments are only applicable to protein (feeding formula) based clogs. Among common emergency room medical procedures, patients rank nasoenteral (NE), (nasogastric [NG]) tube insertion to be one of the most painful (4, 5, 6). Replacing a feeding tube may cause additional patient safety risks, associated medical costs, and additional pain and discomfort to the patient. The most common way to place NE (NG) feeding tubes, is blind insertion at patient bedside, and has a reported 0.5-15% malposition rate (3). Malposition into the trachea may cause pneumothoraxes and possibly death. Several methods exist for verifying accurate tube placement, the most reliable being radiography (3). However, this exposes patients to additional radiation and medical costs.


 valuable nursing resources. Traditional methods to clear clogged feeding tubes are often unsuccessful, potentially leading to tube replacement, and patient discomfort (2,3,4).
valuable nursing resources. Traditional methods to clear clogged feeding tubes are often unsuccessful, potentially leading to tube replacement, and patient discomfort (2,3,4).